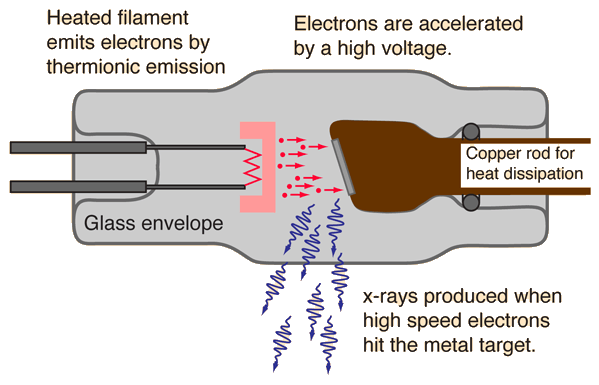
Previously, we were left with this 'hanging' piece of information:
The characteristic X-ray spectrum which consists of sharp peaks of high intensity occurs at specific wavelengths, unaffected by the voltage of the X-ray tube.
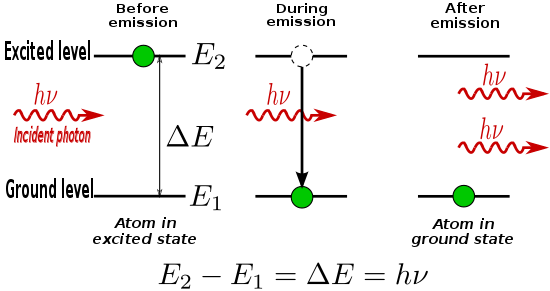
The peaks are a result of the electrons from the cathode knocking out inner shell electrons from the target atoms. When the vacant shells are refilled by free electrons, X-ray photons of specific wavelengths are emitted.Include the two MSpaint sketches of the mechanism behind the characteristic X-ray spectrum here.
The K and L in the previous graph each stand for their characteristic line series peaks respectively.
The K-series peaks consist of Ka and Kb. (assuming Ka = Kalpha and Kb= Kbeta for the sake of convenient typing/editing) These peaks occur when the innermost shell, the K-shell, is refilled by electrons from the outer shells. As a result, X-ray photons corresponding to the series are emitted. The figure above displays the transition responsible of the K-series peaks.
Similarly, The L-series peaks consist of La and Lb, and they occur when the second shell, the L-shell, is refilled by electrons from the outer shells.
The peaks are unique for each target element. In fact, an element can be identified from the peaks.